Today’s news examines many of the ways in which scientists are trying to understand Earth’s atmosphere as well as the atmospheres of exoplanets, mostly in the hopes of figuring out the best way to tell if there is life on another world. Plus, sixty years of fusion power and this week’s What’s Up.
Media
Transcript
Hello and welcome to the Daily Space. I am your host Dr. Pamela Gay.
And I am your host Beth Johnson.
And we are here to put science in your brain.
This first segment of our show is providing the atmosphere for today. No, really. We’re going to share four stories that all look at atmospheres, both here on Earth and on exoplanets.

Let’s start with Earth, where a team of scientists from UC Santa Cruz baked meteorites at 1200 degrees Celsius and then sampled the resulting gases produced using a mass spectrometer. Overall, water was predominant, followed by large amounts of carbon monoxide and carbon dioxide, and then smaller amounts of hydrogen and hydrogen sulfide.
These results are interesting because up until this research, most atmospheric models assumed gases with solar abundances; in other words, heavy on hydrogen and helium. As coauthor Myriam Telus explains: Based on outgassing from meteorites, however, you would expect water vapor to be the dominant gas, followed by carbon monoxide and carbon dioxide. Using solar abundances is fine for large, Jupiter-size planets that acquire their atmospheres from the solar nebula, but smaller planets are thought to get their atmospheres more from outgassing.
Putting it another way, the atmospheres of rocky planets were thought to derive mostly from the gas released due to the intense heat of pulling together all the building blocks and material as well as later volcanic eruptions and outgassing. And this research, which is published in Nature Astronomy, shows that the primitive rocky material in our own solar system contained just such gases as we see in our atmosphere. Side benefit, we can use this information to narrow down our search for certain kinds of exoplanets. Yay for science working as predicted!

Speaking of our own atmosphere, we need to understand how it works if we are to look for similar atmospheric conditions around other planets. Also, we keep talking about this pesky climate change concern, greenhouse gases, and what the future holds if we don’t cool things down. While we have a lot of info about what is going on near the surface of Earth, the upper atmosphere is difficult to understand since the water vapor in the air distorts any data taken, and space instruments haven’t been able to make direct measurements. Additionally, a lot of the data already collected during other observations has been considered noise and discarded. So while climate models predict that temperatures in the upper atmosphere will decrease due to greenhouse gases, scientists were unable to test out their models in reality.
Now, thanks to the SOFIA airborne telescope, we can directly measure the atomic oxygen in the upper atmosphere, and the results match all those snippets of direct measurements from rocket and Space Shuttle instruments as well as the indirect satellite measurements. The confirmation results were published in Nature Communications Earth and Environment using data collected in 2015 during the same flight that observed the Jellyfish nebula we talked about recently. Researchers intend to apply similar analytical methods to data taken during other flights and in different seasons to see just how the atomic oxygen amounts change with location and time.
From oxygen in the atmosphere, we go to rain. A pair of scientists at Harvard recently published a paper in the Journal of Geophysical Research: Planets examining the physics of raindrops and how they might work in different atmospheres. Lead author Robin Wordsworth explains: The humble raindrop is a vital component of the precipitation cycle for all planets. If we understand how individual raindrops behave, we can better represent rainfall in complex climate models.

And it turns out? Raindrops are pretty much raindrops no matter where they fall, although whether or not they reach the surface is a matter of size: too big and the surface tension isn’t enough to hold drops together; too small and the drop evaporates in the atmosphere. What makes a drop “just right”? Well, here you have to combine a few properties — drop shape, falling speed, and evaporation speed.
Drop shapes are pretty similar no matter the material: spherical when small and then squashed flat as they grow. The falling speed is basically a question of the planet’s gravity and the density of the atmosphere. Evaporation speed is the trickstery factor, but taking all that into account led Wordsworth and grad student Kaitlyn Loftus to conclude that no matter the conditions, only a small fraction of drops hit the surface. Again, understanding Earth can help us find the habitable exoplanets we’re looking for, at least so far as life as we know it is considered.
It also turns out that atmospheres can be used to understand the history of an exoplanet, not just its current state. An international team of scientists analyzed the atmosphere of HD 209458b using high-resolution spectra gathered by the Telescopio Nazionale Galileo in La Palma, Spain.

As an exoplanet transits in front of its host star, the light from the star passes through the planet’s atmosphere, exciting the molecules such that they give off energy as they return to their original states, and the light given off by that energy means we can figure out what the molecules are. In this case, the astronomers detected hydrogen cyanide, methane, acetylene, carbon monoxide, ammonia, and a bit of water vapor.
The first four of those are all carbon-based molecules, and that is incredibly unexpected for a planet as close in to its star as HD 209458b is – a mere seven million kilometers away – which leads the team to propose that this exoplanet actually formed much farther out, far beyond the point where water changes from liquid to gas. Coauthor Dr. Siddharth Gandhi notes: There is no way that a planet would form with an atmosphere so rich in carbon if it is within the condensation line of water vapor. At the very hot temperature of this planet (1,500K), if the atmosphere contains all the elements in the same proportion as in the parent star, oxygen should be twice more abundant than carbon and mostly bonded with hydrogen to form water or to carbon to form carbon monoxide.
Remember, this is a giant planet, not a rocky body, so it should have the same composition as its star, and it does not. Every new paper teaches us something more about planetary formation, and we keep inching closer and closer to finding that first truly habitable exoplanet.
Now that we’ve talked about some of the things we can detect in the atmosphere, from atomic oxygen to water vapor to a variety of carbon-based molecules, why do we really care? Sure, we want to find an exoplanet that we can call habitable, at least to life as we know it. We have a sample size of one when it comes to habitable planets, and we’d like to increase that sample. That’s all well and good. But you know and I definitely know that it’s really about finding life. Not just where it could live, but where it does live.
Our previous story showed that we can use spectroscopy to detect the chemical fingerprint of an exoplanet. And my next two stories are going to look at some of the pitfalls we may face when making those directions.
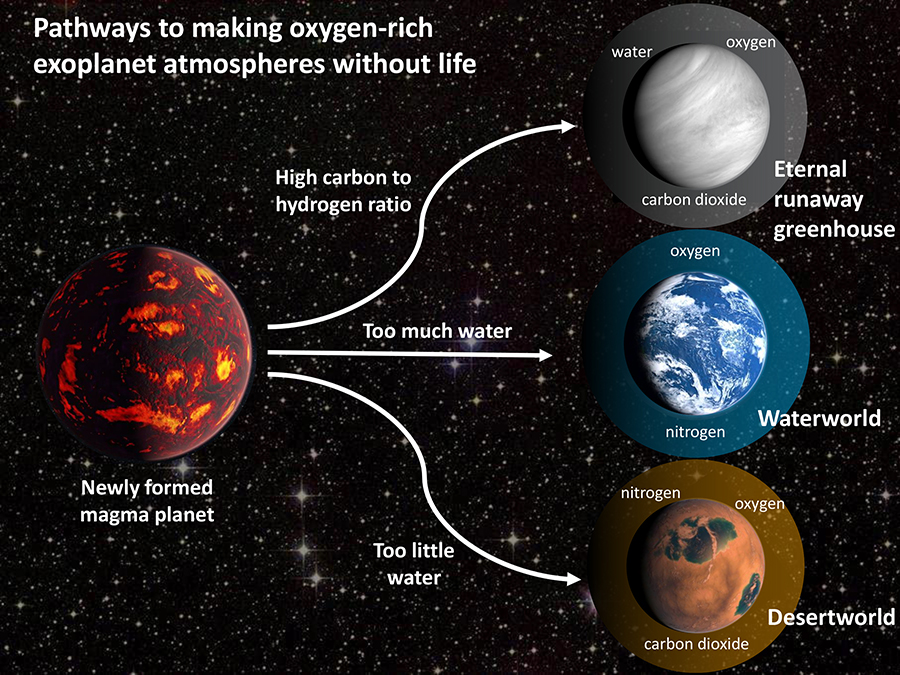
First, let’s talk about oxygen. We’ve mentioned it a few times today, particularly in the context of Earth and our own solar system. We know we have life here. But then we talked about a world’s atmosphere that was far more carbon-based than oxygen-based. This is fine because that planet is too close in and too big to be habitable to the type of life we have here. Sure. Fine. All well and good. Does that mean oxygen is a sure-fire biosignature? No. But new research published this week in AGU Advances seeks to break down how to distinguish between potential “oxygen false positives” and actual signs of life.
The team came up with three different scenarios to potentially explain an abundance of oxygen that isn’t a result of life. Scenario one: oxygen builds up in the upper atmosphere as ultraviolet light breaks apart water molecules in the constituent oxygen and hydrogen. The hydrogen is much lighter, and it tends to escape into space more than the oxygen does. Then add in the outgassing from volcanoes, as mentioned earlier, and now we have carbon monoxide and more hydrogen to add to the mix. Carbon and oxygen like to react, so the oxygen gets pulled into more molecules, and the weathering of rocks on the surface contributes to pulling oxygen out of the atmosphere.
Scenario two: the planet doesn’t have a lot of water, to begin with, so the molten surface of the early planet solidifies quickly before the water that is there condenses. Now you have a steamy atmosphere with a lot of water molecules to break up as before, leaving oxygen behind as the hydrogen, again, escapes.
Then in scenario three, you can have a planet with a runaway greenhouse effect, like Venus, where there is more carbon dioxide than water, and then water cannot condense due to the heat, and so a whole bunch of oxygen-based molecules is just lingering in the atmosphere. It’s all a big, complicated set of processes that add up to one core issue: oxygen comes and goes for more reasons than the respiration of a planet’s inhabitants.
Now here’s the important part, as explained by co-author Jonathan Fortney: There has been a lot of discussion about whether detection of oxygen is ‘enough’ of a sign of life. This work really argues for needing to know the context of your detection. What other molecules are found in addition to oxygen, or not found, and what does that tell you about the planet’s evolution?
Remember those six molecules detected in our last story that told us the planet had to form elsewhere? That’s the kind of chemical fingerprint we’re going to need before claiming any sort of biosignature has been found. It’s more than just oxygen. It’s everything. And the team behind this paper is essentially arguing that we need the right instruments on all these upcoming next-generation telescopes to get the spectra we need from exoplanet atmospheres in order to find that first proper biosignature.
The mere thought of it makes me giddy.
Oh, and there’s another study to help out here. In new research published in Frontiers in Astronomy and Space Sciences, a team of scientists explained how they used computer algorithms to identify the spectral signatures of almost a thousand molecules containing phosphorus. That catalog can now be used to compare future findings and make the identification of atmospheric molecules far easier.
So why phosphorus when I’ve been talking about oxygen all this time? Well, first, there was that whole kerfuffle from last year about detecting phosphine on Venus. The back and forth that played out in the journal was definitely an impetus for this particular modeling project. But also, phosphorus is essential for life, again, as far as we know it. And phosphine was the only molecule we knew how to detect. Now we have 957 other molecules that could maybe, just maybe, indicate life.
Dr. Laura McKemmish of the University of New South Wales, which led the research, explains: Though this new dataset doesn’t yet have the accuracy to enable new detections, it can help prevent misassignments by highlighting the potential for multiple molecular species having similar spectral barcodes – for example, at low resolution with some telescopes, water, and alcohol could be indistinguishable. The data can also be used to rank how easy a molecule is to detect.
Take that phosphine! We’re coming for you. You and 957 of your best friends as well as oxygen and carbon dioxide and water and… well, you get the idea.
As a child of the 80s and 90s, the movie The Saint came out while I was in graduate school, and I had a good giggle at Elisabeth Shue storing the equations for cold fusion in her bra on index cards. There was so much unreal about this (so much), and yet, the idea of cold fusion and the presence of a poetry-spouting Val Kilmer kept my college-age self watching. The stuff of 1990s movies, fusion – cold or hot, take your pick – has been this potential breakthrough, off to the side being worked on, that many have kept hoping would somehow solve the energy crisis.
Nuclear power as we know it relies on fission: the breaking apart of atoms like uranium-235 which can break down into krypton and barium as well as neutrons, light, and energy. Nuclear reactors, when run safely, don’t pollute our environment, but they do create radioactive byproducts that have to be stored and this is problematic.
Fusion, however, works through the combining of safe, lightweight elements, like hydrogen, which release energy when fused into heavy atoms. Objects like our own Sun effectively fuse hydrogen in the high-temperature, high-pressure environment in their cores. Here on Earth, things are less effective, and fusion reactions that produce more energy than they require to maintain just haven’t been produced, yet.
People still keep hoping that fusion and its promise of clean energy are something that will come. In a new review paper in the European Physical Journal, sixty years of data is reviewed to try and understand what factors are at play with plasma-related fusion. Corresponding author Pavel Goncharov explains: Plasma is the dominant state of the visible matter in the present Universe and nuclear fusion powers the stars. The ability to burn deuterium formed at the beginning of the Universe and generate energy represents a new height for mankind.
And with extreme pressure and extreme heat, both of which require extreme energy input, deuterium can be fused. The key is going to be finding a way to fuse things with less energy in and more energy out. Current research is pursuing the possibility of using magnetic fields to confine and compress the plasma. It also builds on the 1951 work by Andrei Sakharov who realized that fast-moving hydrogen ions could collide with slow neutral particles and transfer their charge prior to escaping magnetic confinement. By looking for these fast-moving, neutral escapees, researchers can better understand the ion distributions within the magnetic field and slowly work toward understanding fusion as a power source.
This paper’s authors explain: Three factors played a role in our interest in fusion science. First, it involves multiple branches of fundamental science. Second, this field is of great practical importance. Third, a new clean and abundant energy source is the basis for a better future for mankind. This is an impressive combination.
It’s just a combination that we don’t yet have a clear recipe for combining, and sadly, there is no scientist like Elizabeth Shue’s “Dr. Emma Russel”, who has the answer hidden in their shirt.
What’s Up

Each week we try and bring you some new ideas for some things to go out and see in the sky. Right now a particularly important moon shines for millions of people around the world. In the early evening, a crescent moon can be seen setting in the west, setting a little later every night. This moon is the one that marks this year’s celebration of Ramadan. This holiday spans from the disappearance of the moon through a lunar cycle, until a moon is once again seen in the sky. To those of you who celebrate, Ramadan Mubarek. To everyone else, go out and see the crescent. It’s pretty in the late evening twilight.
Also in the west, Mars hangs bright and red below the Moon, and when you look at it, you’re seeing the only other place in our solar system to have a helicopter.
For those of you who are more into seeing the sky in the morning, this week offers a special show. Right now the Earth is passing through the diffuse cloud of material left scattered by comet c/1861 G1 Thatcher. This comet has a 415-year orbit that carries it out past Eris, so we haven’t actually imaged it, but we know from this meteor shower, the Lyrids, that it left a super impressive spray of debris in the inner solar system.
The Lyrids run from April 16th through April 30th. These meteors will be best on the mornings of April 21st and 22nd, with an average of 5-20 meteors/hour. The meteor’s radiant – that apparent point in the sky where the meteors come from – is in the constellation Lyra just next to the bright star Vega, making it easy to find. The moon will set early enough in the evening that it won’t interfere with observing the Lyrids during the dawn hours.

The appearance of Vega in the morning reminds us that the summer sky is coming. While you’re out there seeing the Lyrids and bright Vega, look lower in the sky to the east. To the left and partway down is Deneb, and to the left and farther down is Altair. This triangle of stars is referred to as the “summer triangle,” and while it is often hidden by light pollution, the path of the Milky Way passes through this part of the sky. We’ll talk more about this asterism of stars in June when it is more of an evening object.
There are also three novae visible using small telescopes, and we’ll get more into those next week. For now though, plan to go out, lay back, and look up.
This has been the Daily Space.
Learn More
These Asteroids Were Baked… For Science!
- UCSC press release
- “Composition of terrestrial exoplanet atmospheres from meteorite outgassing experiments,” Maggie A. Thompson et al., 2021 April 15, Nature Astronomy
SOFIA Measures Atomic Oxygen in Earth’s Atmosphere
- NASA press release
- SOFIA press release
- “Direct measurements of atomic oxygen in the mesosphere and lower thermosphere using terahertz heterodyne spectroscopy,” Heiko Richter et al., 2021 January 26, Nature Communications Earth & Environment
Raindrops Keep Fallin’ on Exoplanets
- Harvard press release
- AGU press release
- “The Physics of Falling Raindrops in Diverse Planetary Atmospheres,” Kaitlyn Loftus Robin and D. Wordsworth, 2021 March 15, JGR: Planets
Chemical Fingerprint Reveals Exoplanet’s Past
- University of Warwick press release
- “Five carbon- and nitrogen-bearing species in a hot giant planet’s atmosphere,” Paolo Giacobbe et al., 2021 April 7, Nature
Oxygen is Not a Reliable Biosignature, Study Finds
- UCSC press release
- “Oxygen False Positives on Habitable Zone Planets Around Sun‐Like Stars,” Joshua Krissansen‐Totton, Jonathan J. Fortney, Francis Nimmo, and Nicholas Wogan, 2021 April 13, AGU Advances
Catalog of Phosphorous Molecule and Their Spectral Signatures Created
- UNSW Sydney press release
- “Computational Infrared Spectroscopy of 958 Phosphorus-Bearing Molecules,” Juan C. Zapata Trujillo et al., 2021 April 8, Frontiers in Astronomy and Space Sciences
Sixty Years of Fission Power and the Hunt for Fusion
- Springer Publishing press release
- “60 Years of neutral particle analysis: from early tokamaks to ITER,” M. P. Petrov et al., 2021 March 19, The European Physical Journal H
What’s Up: Crescent Moon and the Lyrids
- Moon Phases (timeanddate)
- Viewing the Lyrids in 2021 (IMO)
Credits
Written by Pamela Gay, Beth Johnson, and Erik Madaus
Hosted by Pamela Gay and Beth Johnson
Audio and Video Editing by Ally Pelphrey
Content Editing by Beth Johnson
Intro and Outro music by Kevin MacLeod, https://incompetech.com/music/


 We record most shows live, on Twitch. Follow us today to get alerts when we go live.
We record most shows live, on Twitch. Follow us today to get alerts when we go live.