It is possible to buy stickers, sweatshirts, mugs, and all manner of other stuff and things emblazoned with the simple phrase, “We are star stuff”. Carl Sagan popularized this phrase, and it serves as a gentle reminder that all the complex atoms – by which I mean most everything heavier than helium – found their start either in the nuclear core of a star or in the nuclear explosions of a dying star or stars.
But, like with so many things, the truth is much more complicated than the meme.
The Big Bang created hydrogen, helium, and trace amounts of lithium and beryllium. Stars readily fuse carbon, nitrogen, and oxygen, and as you get bigger and bigger, they can create things as complicated as iron in their cores.
Everything heavier than that is formed through drama, and in this segment, we’ll look at just what that drama involves.
Some Atomic Basics
There are two ways to build larger and larger atoms. You can either slam small atoms together and hope the protons and neutrons mostly stick together, or you can flood an atomic nucleus with neutrons and wait for some neutrons to decay into protons and give you that larger atomic number.
Atoms are defined by the number of protons in their core. Carbon has six protons; iron has 26. Plutonium has 94. The variety of an atom is further defined by how many neutrons an atom has. For instance, the variety of carbon we use to measure the age of archeological finds has eight neutrons. Since six plus eight is fourteen, we call that form of carbon “carbon-14”. Similarly, the kind of plutonium used in the radioisotope thermoelectric generator (RTG) in spacecraft like Cassini has 144 neutrons and since 144 plus 94 is 238, we call it plutonium-238.
Different atoms – such as carbon, plutonium, and lead – have different possible numbers of neutrons. While we know of 118 different possible atoms we can line up on the periodic table, those atoms collectively come in 270 different stable varieties, and we’ve observed more than 3,000 different nuclei if you include the unstable varieties.

This difference between stable and unstable all comes down to how the atoms are constructed.
Within the atom, the protons and neutrons are arranged in shells. And like any DIY project you might have built, the atomic nuclei are most stable when a given shell is full, and if things are built wrong in just the right way, chunks will pop off when given the opportunity. This is where we see some atoms fall apart while others, like iron, can seemingly hang around for ages.
The sizes of the shells the atoms are filling with protons and neutrons can take some mighty jumps. From a nice stable full shell at 50 particles, we jump up to 82 and then 126 and 184 particles. Looking at the periodic table, this is the jump from tin to lead, and then to a place where we have not yet found atoms.
It had been predicted that there could be – should be – an island of stable atoms if we can somehow slam together a big enough atom and reach that next full shell. The more we explore our universe and the more we create things in labs, the more it looks like we will find more unstable atoms: ones that will last a half minute, even a few hours. But we won’t find stable atoms. For instance, Lawrence Berkeley National Laboratory and the Joint Institute for Nuclear Research created the element lawrencium. With 103 Protons, it has no fully stable version, but lawrencium-266, with 163 neutrons, is stable for just over 22 hours and has been detected outside the lab; it was detected as part of a neutron star merger observed in 2017. This particular element has enough protons to create another orbital shell for its outermost protons but not a full shell. Its neutron number is also creeping toward the next magical number at 184, and there are indications that the atom is getting more stable with the added neutrons, but…
In a new paper appearing in Nature Review Physics, scientists led by Odile Smits admit that we’re not going to find a new row of stable elements to add to our periodic table. It’s unclear exactly why that mystical island of stability doesn’t seem to be there, but if it was, we should be seeing signs of it, and we aren’t.
This is where it may all come down to timing — enough protons and neutrons have to pile up in one place to form a giant atom, and they have to come together so fast that smaller, stable elements don’t have a chance to form first.
When an Atom Meets an Atom, You Get Fusion
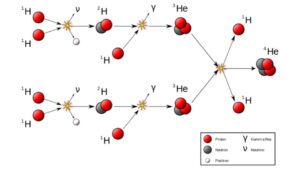
Atoms form in a couple of different ways. The most familiar way is to smash small atoms together, and at a certain level, a rogue proton is a hydrogen nucleus. You have to smash small atoms together so that they have an opportunity to form heavier atoms. Smush enough hydrogen atoms together, and you’ll eventually get helium. Smush enough helium together, and you’ll eventually start to get beryllium and carbon. This is the stuff of stars, and you, my wonderful audience, are mostly carbon which is the stuff of stars.
A Bombarded Atom Just Decays
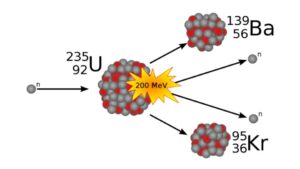
The other way to grow atoms is to take advantage of the instability of neutrons. These chargeless bits of matter will readily split into a proton, electron, and antineutrino. Leave one alone on an imaginary shelf and boom — give it enough time, and its 10-minute half-life is going to earn you a small bundle of… not neutrons.
In supernova explosions and the mergers of neutron stars, you can end up with rapid outflows of neutrons. The flux of these particles can quickly drive the number of neutrons in an atomic core to ludicrous levels, and then they decay, which gives us really cool heavier atoms. For instance, in merging neutron stars, a regular, totally stable atom like iron with 26 protons and 30 neutrons can get bombarded with neutrons until it has transformed into something like gold, with 79 protons and 118 neutrons. And gold is just the start of what can be created. But at a certain point, it is just starting to look like this rapid neutron bombardment process can’t get us to that wished-for island of stable massive atoms beyond the edge of our known periodic table. We will probably keep finding bigger atoms as technology advances, but the universe isn’t going to be the one to provide.
Not All Atoms Form Fast
As a side note for the completionists in our audience: while the vast majority of our heaviest elements form either in supernovae or a neutron star merger, not all atoms require violence to grow. In dying low-mass stars, neutrons can – one at a time – crash into atomic nuclei, and this slow bombardment can cause atoms to creep up one atomic number at a time through the periodic table. It’s slow, but it gets the job done.
That’s it. That’s how atoms form… and don’t form as big as we’d hoped.
